What Element Has the Electron Configuration 1s22s22p63s23p3
Correct option is A. Phosphorus The 1s orbital Given that the electron configuration for phosphorus is 1s22s22p63s23p3 answer the following questions.

Solved The Lewis Structure Below Represents The Valence Chegg Com
I want the order of Z not the order of filling.

. How many valence electrons does 1s22s22p63s23p3. The electron configuration 1s22s22p63s23p2 is the element Silicon. Period 3 element phosphorus has 5 electrons P.
Jun 02 2021 1s22s22p63s23p3 is the original electron configuration for PhosphorusIt will gain three electrons leaving it with the same configuration as Ar or 1s22s22p63s23p6 What element has the ground-state. Which element has the electron configuration 1s22s22p63s23p3. What element has the electron configuration of 1s22s22p63s23p3.
Which element has the following electron configuration 1s2 2s2 2p3. How many valence electrons does 1s22s22p63s23p3. Phosphorus has the electron configuration 1s22s22p63s23p3.
How Many Valence Electrons Does 1S22S22P63S23P3. How many valence electrons does group 1 have. Electronic configuration of chemical elements is the classification and ordering of electrons around the nucleus of an atom in reference to their energy levels.
How many unpaired electrons are in an atom of chromium Cr in its ground-state. Valence electrons are outer shell electrons with an atom and can participate in the formation of chemical bonds. This problem has been solved.
Which ion has the ground-state electron configuration. What element has the electron configuration. So an atom cant have no electrons as it by definition has protons and to be neutral must have electrons.
The atomic number and the group number of the element X which is just below the given element in the periodic table are respectively. The electronic configuration of an element is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3. What is the electron configuration for the Potassium K atom in its ground-state.
The electronic configuration of an element is 1s22s22p63s23p3. Part 2 1 The element with an electron configuration of 1s22s22p63s23p64s23d6 is in group and period. _____ What is the ions charge.
2 The element with an electron configuration of 1s22s22p63s23p3 is in group and period. Phosphorus has electron configuration 1s22s22p63s23p3. See the answer See.
Which of the following elements would exhibit the greatest shielding effect. Therefore the Chlorine electron configuration will be 1s22s22p63s23p5. The Period 3 element with 5 valence electrons is phosphorus P.
What element has the electron configuration 1s22s22p63s23p3. Since the 3s if now full well move to the 3p where well place the remaining five electrons. Which is the atomic number of the element which is just below the above element in the periodic table.
2 electrons in the 3s subshell and 3 electrons in the 3p subshell. Explicit or complete electrons are listed 1s22s22p63s23p3. The complete electron configuration is 1s22s22p63s23p3.
What element has the electron configuration of 1s22s22p63s23p64s23d3. Therefore the Phosphorus electron configuration will be 1s22s22p63s23p3. What is the atomic number of the element which is just below the above-given element in the periodic table.
The electronic configuration of an element is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3. To figure this out the element with the electron config of we first count the electrons. You can have an ion such as a hydrogen ion you might call it a proton.
Electron Affinity EA is the energy change that occurs when 1 mol of electrons is added to 1 mol of gaseous atoms or ions. 1s22s22p63s23p3 is the original electron configuration for PhosphorusIt will gain three electrons leaving it with the same configuration as Ar or 1s22s22p63s23p6. Which element has the electron configuration 1s22s22p63s23p3.
What are valence electrons and what do they have to do with bonding. Expert answeredjerry06Points 85659 User. If an element has atoms in their.
Which element has the electron configuration 1s22s22p63s23p3. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. How many valence electrons does group 2 have.
1s22s22p63s23p3 contains only 5 valence electrons in its outermost shell. A one mol drop of electrons from a pleon to an ions influences electron affinity which results in energy changes. Z 15 with P.
Check all possible answers. An atom that has the following electron configuration. What is the electron configuration of P.
Correct option is C. Those are the small number placed at the top after the letters. Well put six in the 2p orbital and then put the next two electrons in the 3s.
Does phosphorus have stable electronic configuration. Can an element have 0 electrons. The highest-numbered shell is the third shell 3s23p3.
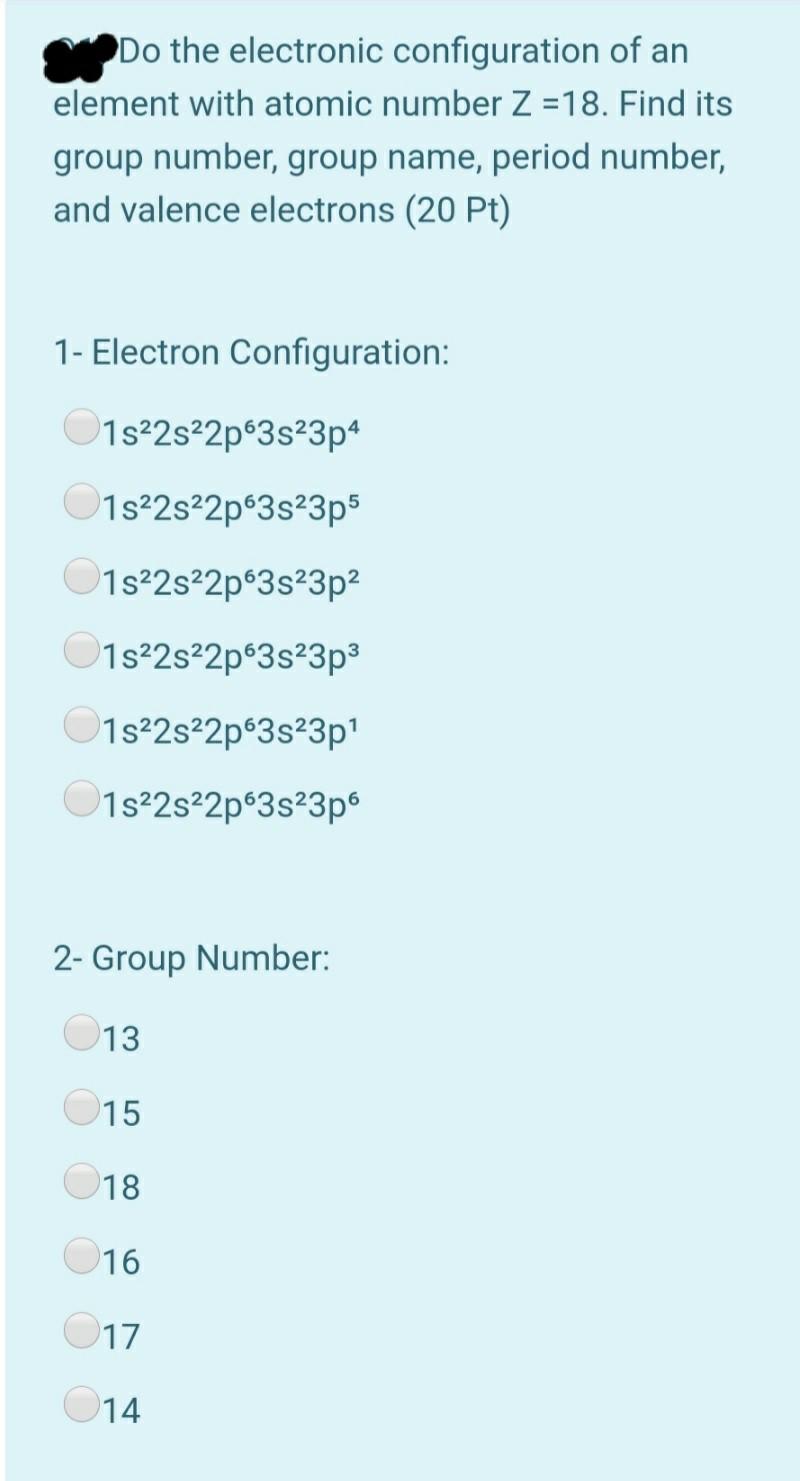
Solved Do The Electronic Configuration Of An Element With Chegg Com
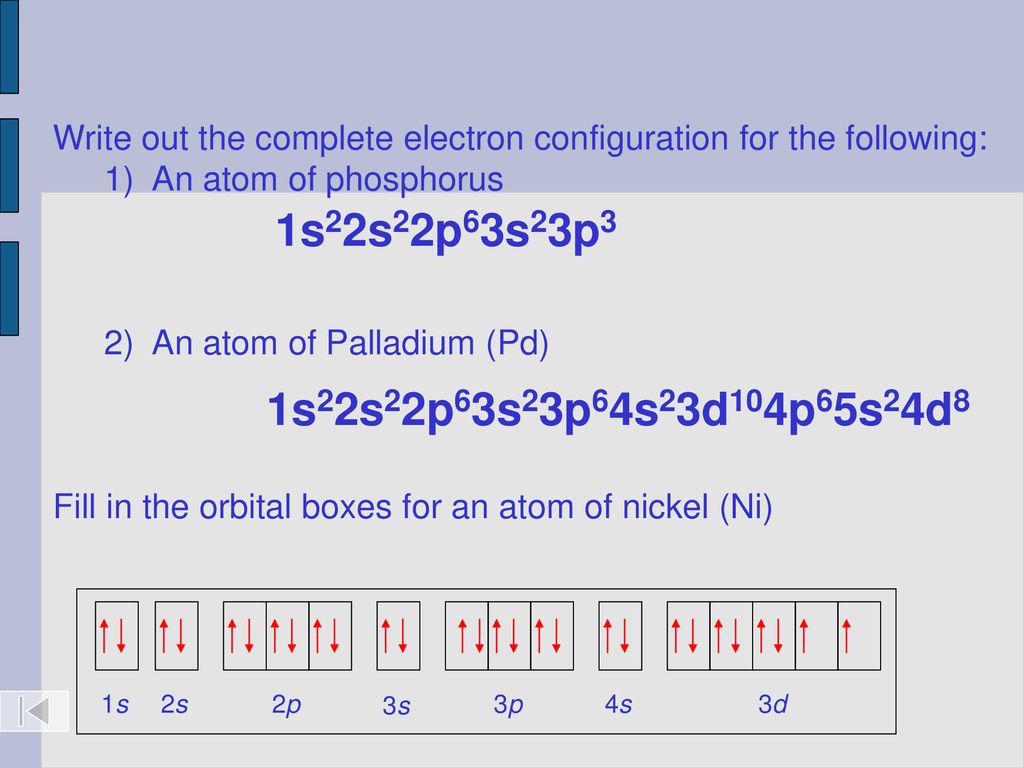
Today S Lesson Electron Hotels Ppt Download

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 Youtube
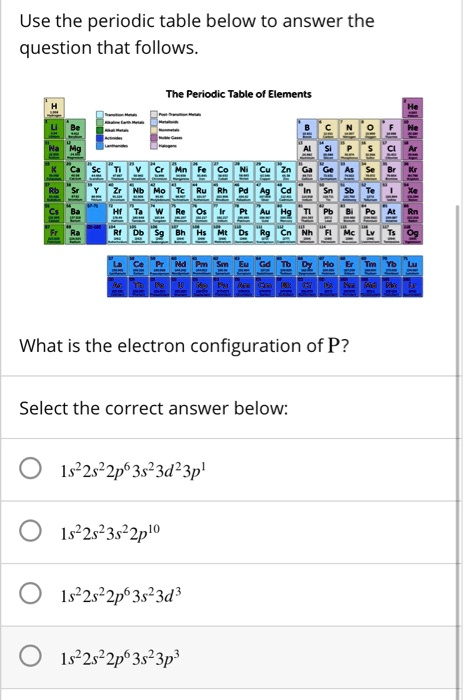
Solved Use The Periodic Table Below To Answer The Question That Follows The Periodic Table Ol Elements What Is The Electron Configuration Of P Select The Correct Answer Below 1s22s22p63523d23p 1s22523522pl0 1s22822p63523d 1s22s22p63s23p3

Electronic Structure Of Atoms Electron Configurations General Chemistry I
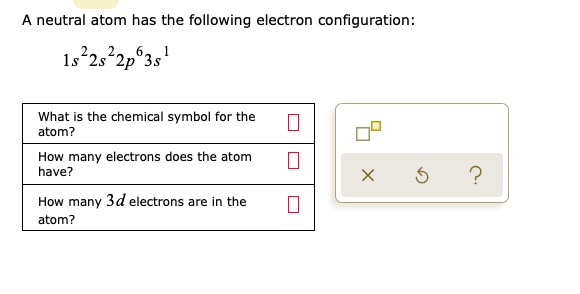
Solved A Neutral Atom Has The Following Electron Configuration 1s 22s22p635 What Is The Chemical Symbol For The Atom How Many Electrons Does The Atom Have 2 How Many 3d Electrons Are In

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p3 Youtube
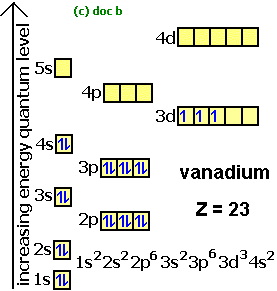
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Electronic Configurations Ppt Download

Solved Give The Symbol Of The Atom With The Electron Chegg Com

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p3 Youtube

Solved 5 Which Elements Are Represented By The Following Chegg Com

Solved Give The Name Of An Element That Has The Same Number Of Valence Electrons As The Element With The Electron Configuration 1s22s22p63s23p3 The Answer Is Not Phosphorus Give The Name And
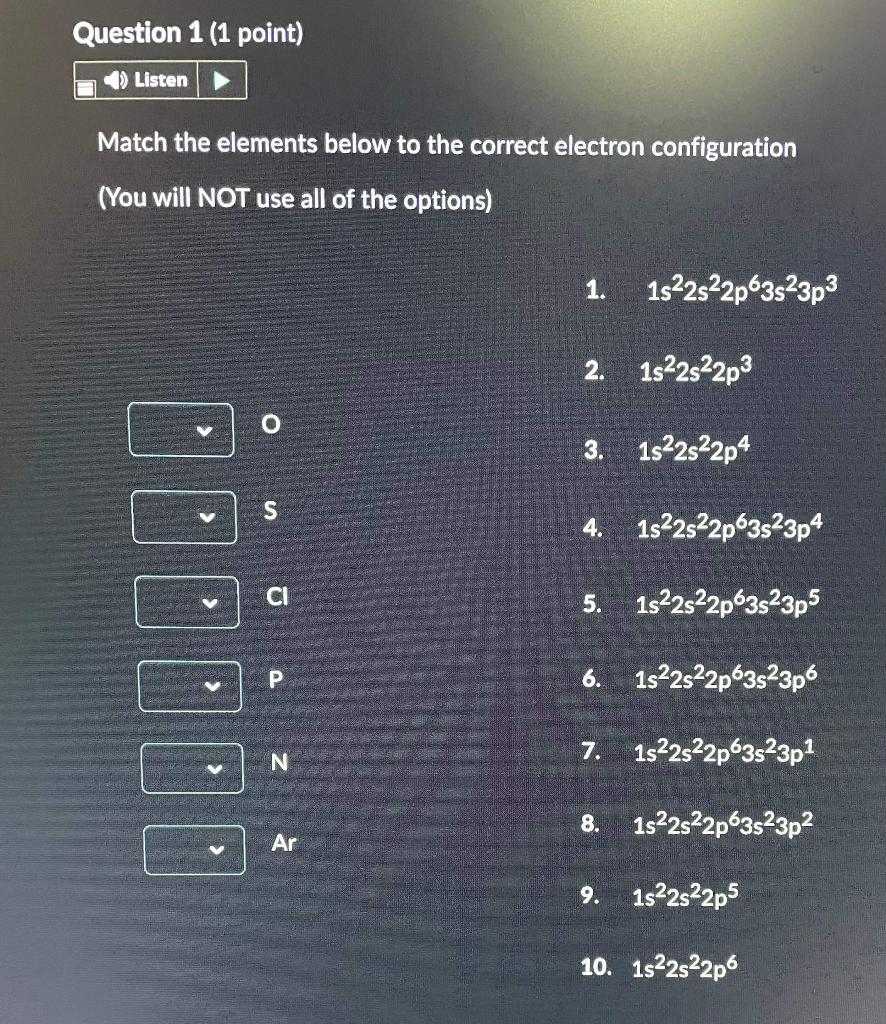
Solved Need Help With These 4 Questions 1 Match The Chegg Com
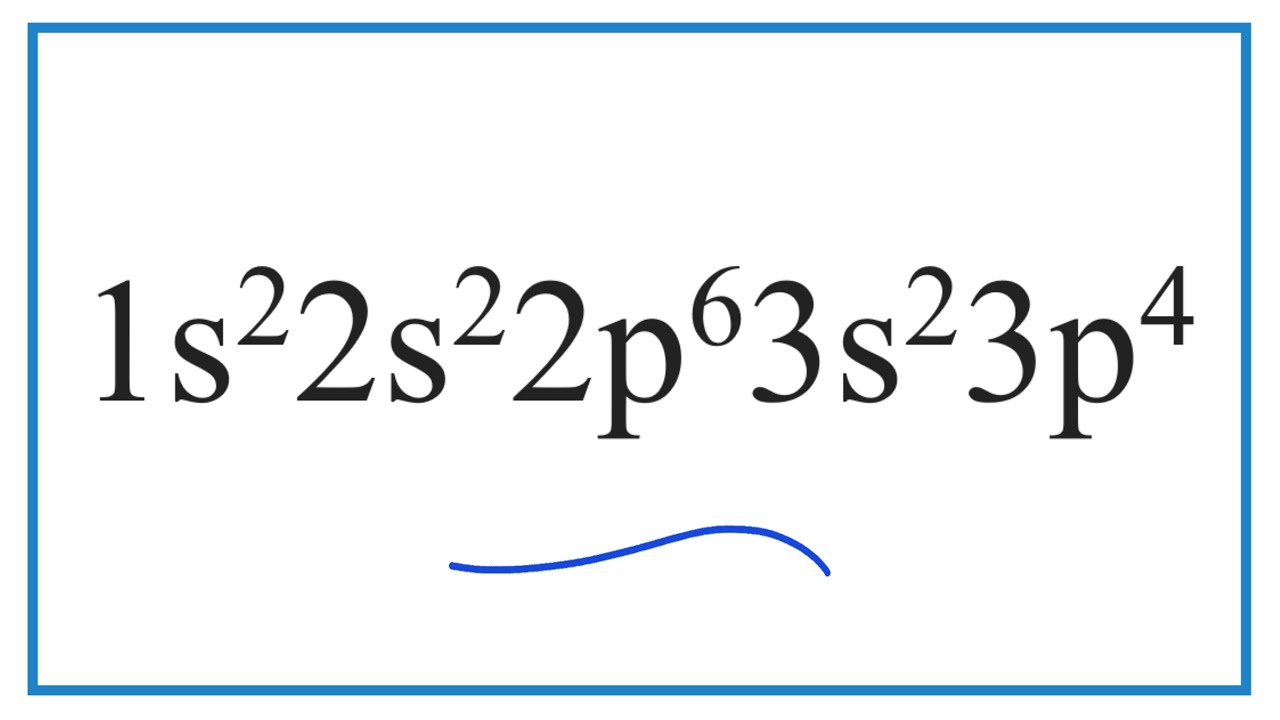
What Element Has The Electron Configuration 1s22s22p63s23p4 Lisbdnet Com
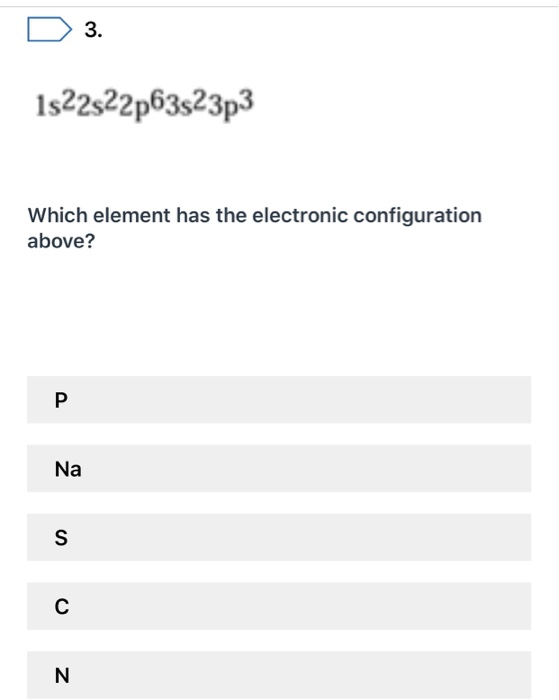
Solved 3 1s22s22p63s23p3 Which Element Has The Electronic Chegg Com

Solved The Lewis Structure Below Represents The Valence Chegg Com
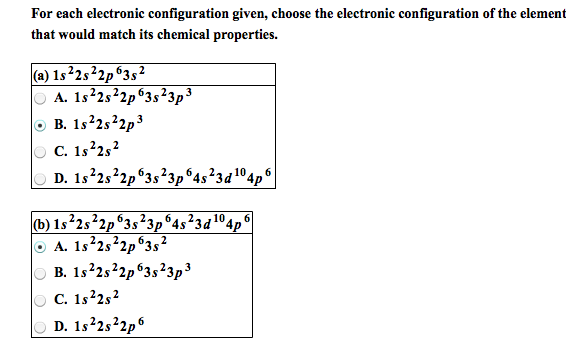
Solved For Each Electronic Configuration Given Choose The Chegg Com
Comments
Post a Comment